Background: The use of a von Willebrand factor (VWF) concentrate for long-term prophylaxis is recommended for individuals with von Willebrand disease (VWD) who have a history of frequent and severe bleeds, regardless of their age or the type of VWD. Bleeding from the nose (epistaxis) is one of the most common types of bleeding in VWD patients. The WIL-31 study demonstrated the efficacy of prophylaxis with a plasma-derived VWF/factor VIII (pdVWF/FVIII) concentrate containing VWF and FVIII in a 1:1 activity ratio (wilate ®) in adults and children with VWD of all types. The primary endpoint was met, showing a decrease of 84% in the mean total annualized bleeding rate (ABR) compared with prior on-demand treatment.
Aims: To investigate the efficacy of regular prophylaxis with a pdVWF/FVIII concentrate in reducing nose bleeds in people with VWD, compared with previous on-demand treatment.
Methods: WIL-31 (NCT04052698) was a prospective, non-controlled, international, multicenter phase 3 trial that enrolled male/female patients, aged ≥6 years with VWD type 1 (VWF:RCo <30 IU/dL), type 2 (except 2N) or type 3. Prior to entering the WIL-31 study, all patients had received on-demand treatment with a VWF-containing product during a 6-month, prospective, observational, run-in study (WIL-29); patients who experienced at least 6 bleeding episodes, excluding menstrual bleeds, of which ≥2 were treated with a VWF-containing product, were eligible to enter WIL-31. Patients in WIL-31 received regular prophylaxis with pdVWF/FVIII 2-3 times per week at a dose of 20-40 IU/kg for 12 months. In this post-hoc analysis, nose bleeds during WIL-29/-31 were described, and the mean ABRs were compared.
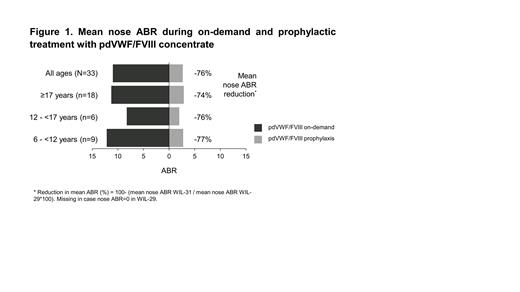
Results: The study population included 33 patients with a median (range) age of 18 (7-61) years; 9 (27.3%) patients were 6-11 years old, 6 (18.2%) were 12-16 years old, and 18 (54.5%) were ≥17 years old. All VWD types were represented: type 1 (n=6), type 2 (n=5); and type 3 (n=22). There were 173 breakthrough bleeds in total during WIL-31, of which nose bleeds were the most common (89/173; 51.4%). Nose bleeds occurred in 16 of 33 patients during prophylaxis vs 26 of 33 during on-demand treatment. Of the 89 nose bleeds, 17 were traumatic, 69 spontaneous, and 3 due to allergic reaction (not product related). The mean number of spontaneous nose bleeds per patient during prophylaxis was 2.1, 2.3, 1.2, and 2.2, for the overall population, and the 6-11, 12-16, and ≥17 years age groups, respectively. Eight nose bleeds (9.0%) were major (all in the same patient). The mean nose ABR was reduced by 76% during prophylaxis vs on-demand treatment. The reduction was consistent across age groups (74-77%, Figure 1) but not across VWD types, with mean nose ABR reductions of 48, 89, and 80% for VWD type 1, type 2, and type 3 patients, respectively. During prophylaxis, a higher number of VWD type 3 patients, compared with type 1 and type 2 patients, experienced nose bleeds (12 vs 3 vs 1). The mean duration of nose bleeds decreased during prophylaxis vs on-demand for 12-17 years old, ≥17 years old, type 2 and type 3 VWD patients, but increased for 6-11 years old and severe type 1 patients. No relationship was observed between the number of nose bleeds and patient age (R 2=0.082). The majority (80.9%; 72/89) of nose bleeds were treated with pdVWF/FVIII with 86.1% (62/72) requiring only a single infusion; two required concomitant treatment with tranexamic acid. All treated nose bleeds were rated as “successfully treated”.
Conclusion: The data indicate thatprophylaxis with pdVWF/FVIII was effective at reducing nose bleeds in patients with VWD type 2 and 3, and across all age groups.
OffLabel Disclosure:
Boban:Octapharma: Honoraria, Research Funding; Amgen: Honoraria; Astra Zeneca: Honoraria; Bayer: Honoraria; CSL Behring: Honoraria; Novo Nordisk: Honoraria; Pfizer: Honoraria; Roche: Honoraria; Sobi: Honoraria; Swixx: Honoraria; Takeda: Honoraria. Sidonio:UniQure: Honoraria; Biomarin: Honoraria; Spark: Honoraria; Novo Nordisk: Honoraria; Bayer: Honoraria; Guardian Therapeutics: Honoraria; Takeda: Honoraria, Research Funding; Octapharma: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Pfizer: Honoraria. Dubey:Octapharma: Other: Clinical study investigator . Khayat:Octapharma: Honoraria; CSL Behring: Honoraria; LFB: Honoraria.
This study is investigating the use of a VWF / FVIII concentrate for the prevention of bleeds in patients with von Willebrand disease of any type

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal